If your practice does lab tests, you need to know about CLIA waivers not only to meet the requirements of the law, but also to make sure you get paid properly and stay in order.
The Clinical Laboratory Improvement Amendments (CLIA), established in 1988, set the rules for lab testing on humans in the U.S., ensuring test results are accurate, timely, and reliable.
But here’s where it matters for you: CLIA waivers directly affect reimbursement, coding, and whether you can offer convenient point-of-care testing. In this blog, we’ll break down what CLIA waivers mean, how they impact medical billing, and what you need to know to avoid costly mistakes and maximize revenue.
What is a CLIA Waiver?
A CLIA Waiver refers to a specific classification under the broader CLIA regulations that allows certain laboratory tests to be performed with minimal risk of error. These waived tests are considered simple to administer and have a low probability of producing an incorrect result.
The waiver permits non-laboratory personnel, such as clinicians in physician offices or clinics, to conduct basic testing without the need for more stringent laboratory certifications.
CLIA Certification vs. CLIA Waiver
CLIA certification and waiver reside under the same regulatory framework but have different purposes.
Laboratories that do moderate to high-complexity tests need to be CLIA-certified, which includes strict rules, quality control procedures, and inspections.
A CLIA permit, on the other hand, is given for tests that aren’t very complicated, and the application and approval process is sped up. Facilities that have a waiver don’t have to follow as many of the strict rules that apply to higher-level labs.
Types of Tests Allowed Under a CLIA Waiver
CLIA-waived tests are generally designed for point-of-care use and do not require extensive laboratory infrastructure or highly trained personnel. Common waived tests include:
- Blood glucose monitoring
- Urinalysis dipstick or tablet tests
- Pregnancy tests using urine
- Fecal occult blood tests
- Rapid strep and influenza tests
- Cholesterol testing devices approved for home use
These tests are FDA-approved for home use or designed to be error-proof and simple, thereby qualifying for CLIA waiver status.
Importance of CLIA Numbers in Billing and Reimbursement
Following government rules is very important for healthcare to provide good care and make sure bills are correct. CMS is in charge of the Clinical Laboratory Improvement Amendments (CLIA), which make sure that labs follow the rules for giving correct test results.
To bill Medicare, Medicaid, and many private insurers for lab work, you need a valid CLIA number. Claims that don’t have a CLIA certification are often turned down, which slows down the revenue stream.
Facilities that do waived, moderate, or high-complexity tests need to be CLIA-certified, and this information must be included in insurance reports in order to get paid back. For compliance and reimbursement, claims must have the right CLIA paperwork.
Applying for a CLIA Waiver
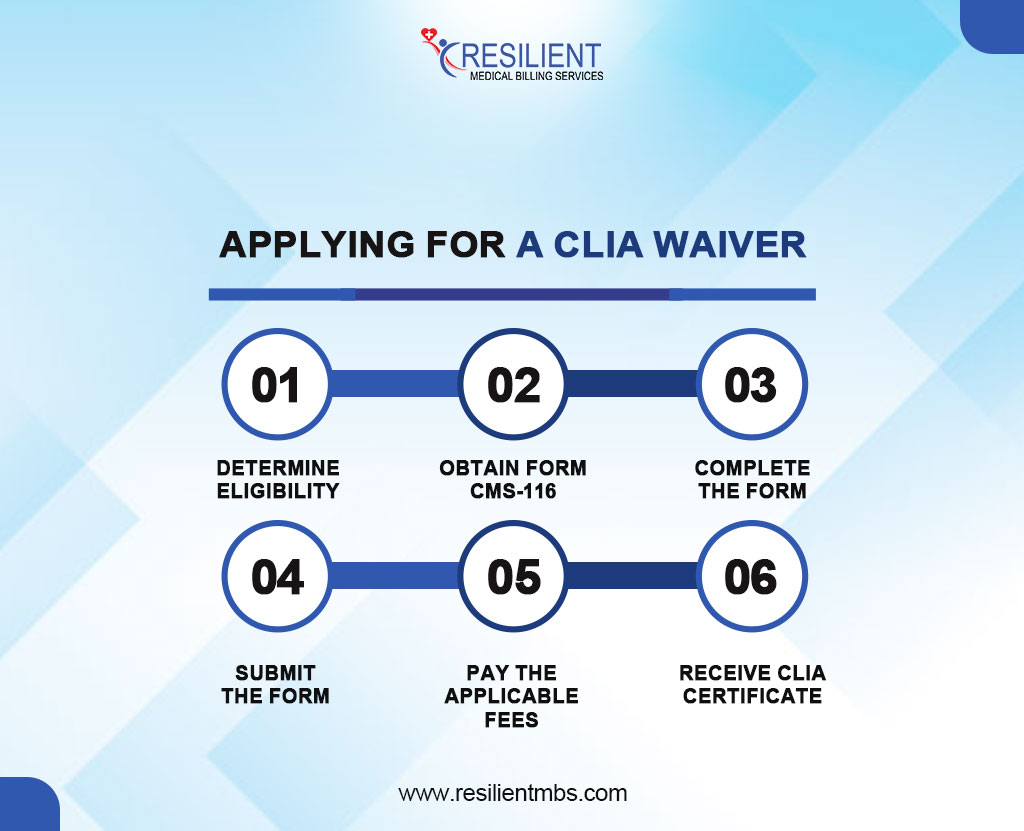
Step-by-Step Process for CLIA Certification Application
- Determine Eligibility: Confirm if your practice or facility will only perform CLIA-waived tests to qualify for a CLIA Certificate of Waiver.
- Obtain Form CMS-116: Get the official CLIA application form to begin the certification process.
- Complete the Form: Fill in necessary details like the laboratory’s name, address, ownership, test types, and operating hours.
- Submit the Form: Send the completed form to the appropriate state agency for processing.
- Pay Applicable Fees: After submission, you’ll receive a fee coupon from CMS. Make the required payment.
- Receive CLIA Certificate: Once approved and payment confirmed, you will receive the CLIA Certificate of Waiver and a unique CLIA number.
Where and How to Submit the Application for CLIA Waiver
The CMS-116 form must be submitted to the state agency overseeing CLIA in the applicant’s location. CMS provides a list of state contacts on its website to help providers identify the correct submission address or email. Ensure all sections are accurately completed, as incomplete applications may cause delays.
Form CMS-116 includes several sections:
Section I: Laboratory director and contact details.
Section II: Certification type being requested.
Section III: Test menu and methodologies.
Section IV: Ownership and billing information.
Sections V–VII: Signatures and compliance attestation.
Accuracy and completeness are important. After approval, providers are authorized to conduct waived testing under CLIA.
What is a CLIA Number in Medical Billing?
A CLIA number refers to the unique identification number issued by the Centers for Medicare & Medicaid Services (CMS) under the Clinical Laboratory Improvement Amendments (CLIA) of 1988.
This number is required for all laboratories and facilities that perform testing on human specimens for health assessment, diagnosis, prevention, or treatment of diseases. The CLIA number is very important when sending requests for lab services to Medicare and other insurance companies for medical billing.
When billing for laboratory procedures, particularly waived tests, the CLIA number must be included on the CMS-1500 or UB-04 claim forms. Insurance carriers use it to verify the testing facility’s certification. Missing or incorrect CLIA numbers can lead to delayed reimbursements or claim denials.
How to Obtain and Verify a CLIA Number
Providers can obtain a CLIA number by applying through the CMS CLIA Program using Form CMS-116, paying applicable fees, and selecting the appropriate certification level. Once approved, CMS issues a CLIA certificate with a unique number.
To verify the number, use the CMS CLIA Laboratory Lookup tool to confirm its validity and ensure it matches the lab’s testing level.
Common Questions and Billing Tips
Q. What is a CLIA number in medical billing?
A CLIA number is a unique identifier for labs performing clinical tests. It’s required for billing laboratory services under the CLIA program, and claims without it may be denied.
Q. When is a CLIA Waiver required for billing?
A CLIA Waiver is needed for facilities performing simple, FDA-approved tests. Billing must include the CLIA number, and tests must align with the waiver certificate.
Q. How do I apply for a CLIA Waiver?
Complete CMS Form 116 and submit it to the state agency with the waiver option and fees. Once approved, the provider receives a CLIA number.
Q. Can billing errors occur if the CLIA number is missing or incorrect?
Yes, claims may be denied or rejected if the CLIA number is missing or incorrect. Correct inclusion ensures proper reimbursement.
Q. Where can I find the official CLIA Waiver form?
The CLIA waiver form (CMS Form 116) is available on the CMS website or through the state agency overseeing labs.
Best Practices for Ensuring Accurate Medical Billing with CLIA-Related Services
- Verify CLIA Status
- Use Correct Test Codes
- Include the CLIA Number
- Stay Current on Regulations
- Educate Billing Staff
Final Words
A solid understanding of CLIA Waivers is vital for healthcare providers and billing professionals. These waivers allow providers to bill for low-complexity lab tests, meeting regulatory requirements. CLIA certifications and waivers must be completed correctly to avoid claim denials and accelerate reimbursement.
Call Resilient MBS LLC right away for trustworthy CLIA certification, application, and claims submission assistance. We can help you comply, minimize denials, and improve revenue cycle performance.
See our website or get in touch to find out more about our medical billing and compliance offerings.